Want to know more about toxicological assessment
and our collaboration with Flashtox? Drop us a line
The food packaging sector is characterized by stringent regulations, due to the significant health risks that could arise from errors or flaws in product or component development. A solid paper and cardboard manufacturing company has decided to study a new compound for the internal protective coatings of its food packaging (FCM) to ensure the safety of the packaging required by a new client company, a baby food producer.

The baby food sector is heavily impacted by the renewed attention of the purchasing decision-maker towards the type of packaging and the possibility of recycling, making the opportunity to use paper and cardboard packaging a concrete competitive advantage for baby food manufacturers.
This focus, particularly in Italy, has in no way diminished the demand for product safety guarantees, given the vulnerability of the end users (infants aged 0–12 months and children aged 1–3 years). For this reason, the FCM manufacturing company has invested in R&D to identify a coating solution for its packaging that is toxicologically safer even at high temperatures, which is fundamental in the production and packaging of homogenized products and baby food.
Objective of the toxicological assessment:
Assessment of multiple exposure to a mixture placed on the market and intended for consumers, with particular reference to children, pursuant to Articles 6 and 8 of Regulation (EU) 2023/988 using computational methodologies (Vega Toolkit).
The Advantage of this compound
One of the main advantages for a company in using this UVCB compound is its thermal stability up to 240 °C. This characteristic reduces the risk of substance release during production and use, helping to contain the environmental impact, provided that the disposal of the FCM also occurs correctly.
Furthermore, the chemical structure provides photochemical stability (presence of conjugated double bonds), ensuring color stability even in environments exposed to sunlight. The presence of alkyl amines helps it adhere to paper and cardboard, improving the technical performance of the final product.
Molecule subjected to computational toxicological analysis:
AMINES,C12-14-TERT-ALKYL,BIS[2-[(4,5-DIHYDRO-3-METHYL-5-OXO-1-PHENYL-1H-PYRAZOL-4-YL)AZO]BENZOATO(2-)]CHROMATE(1-)
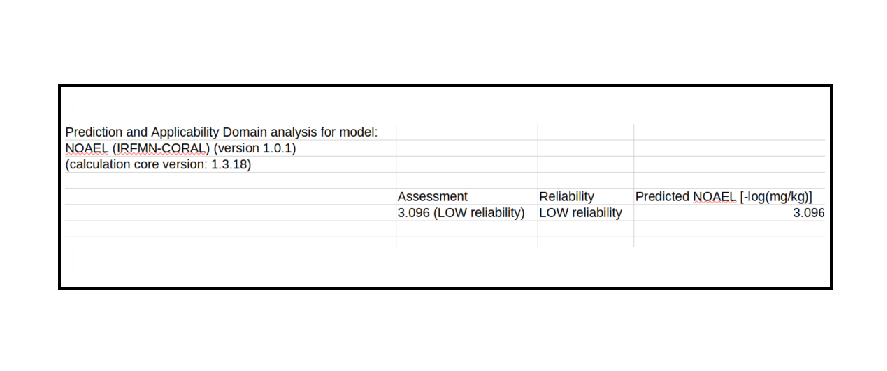
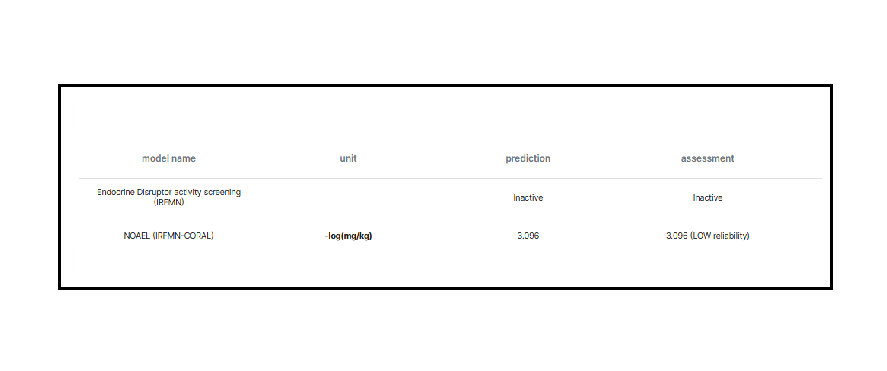
In order to assess multiple exposure to the substances contained in the mixture under examination, the MOET (Margin of Exposure Multiple) method was used. To calculate the MOET, it is necessary to derive MOE (Margin of Exposure) values for each component of the mixture. The determination of MOE is based on the use of POD (Point of Departure), i.e., toxicological descriptors obtained from animal studies. However, the previously reported substance does not have adequate PODs in the literature for this calculation purpose; therefore, the NOAEL (No Observed Adverse Effect Level) was estimated using the VEGA toolkit (IRFMN-CORAL).
COMMENT: In the absence of specific quantitative exposure data for the PC26 category (paper and board treatment products), an estimated chronic exposure value for the PC9a category was used, as the application method and professional use are comparable. The initial exposure estimate (4.9 mg/kg bw/day) refers to the global migration of substances from the hypothetical PC9a category product (Coatings, paints, thinners, paint removers – Solvent rich, high solid, water borne paint – SEE SOURCE). Taking as an example Ministerial Decree of March 21, 1973, which establishes the composition and purity requirements for FCMs, the target substance should not contribute more than approximately 10% of the total migration. Therefore, the maximum estimated exposure was proportionally reduced to 0.49 mg/kg bw/day, providing a more conservative estimate for the substance under examination. Source: TR126: Guidance for Effective Use of Human Exposure Data in Risk Assessment of Chemicals (Table 2: Reasonable worst-case consumer exposure to a highly volatile solvent assessed using the EGRET v.1.0 model. Product categories highlighted in grey were excluded from aggregate exposure assessment).
This approach allowed the identification of the most suitable POD for the MOE calculation, ensuring an accurate assessment of multiple exposures to the substances in the mixture.
COMMENT – Result interpretation: A result much lower than 100 does not necessarily indicate that the exposed target population is at risk, but it is plausible to hypothesize that specific risk management measures could promote better consumer protection.
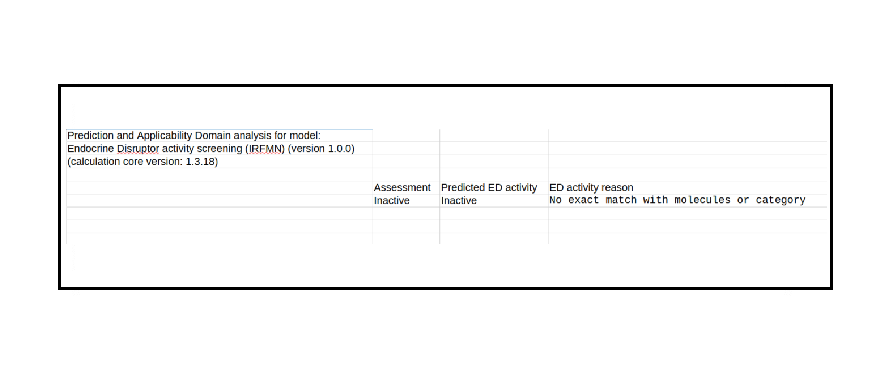
The toxicological assessments carried out aim to be cautious, especially for the most susceptible population groups. The VEGA toolkit was also used to analyze the potential endocrine disruptor activity (Reg. (EU) 2023/707) of the substance in question. The application of the VEGA toolkit contributed to integrating the toxicological report, filling any gaps deriving from the lack of available data in the literature or providing a starting point for further toxicological investigations.
Junes and Vega Toolkit represent an important evolution compared to classical methodologies in the regulatory field, especially regarding data management and the generation of technical and scientific documentation. They allow for rapid and reliable preliminary toxicity assessments thanks to validated QSAR models. They automate predictive analysis and support the drafting of regulatory dossiers, reducing time and costs compared to traditional in vitro or in vivo tests.
The results obtained regarding the No Observed Adverse Effect Level (NOAEL) as predicted by the VEGA Toolkit allowed the calculation of the POD, with which the MOE levels guaranteed by the molecule were estimated, making it a valid candidate for the evaluation of a new coating for infant food packaging.

Want to know more about toxicological assessment
and our collaboration with Flashtox? Drop us a line
This case study aims to illustrate, by way of example, the methodological approach to risk characterization of a mixture intended for use in food contact materials and articles.
Given the structural complexity of the substance examined and the variability of the use scenario, the results presented do not constitute an exhaustive toxicological assessment. The exposure estimates and considerations proposed are based on partial data, adopted for demonstrative purposes and should not be used as a reference for operational or regulatory decisions.
To ensure appropriate chemical risk management and full regulatory compliance, a personalized and in-depth technical-scientific evaluation is always necessary.For technical insights or to receive support in the evaluation of substances, mixtures or materials intended for food use, you can contact us directly: info@flashtox.com.